Peer-reviewed Scientific Research Articles on MG Beta Glucan Preparation and Immune Potentiation
The following articles published in recent years are authored by the research team at the University of Nevada School of Medicine, School of Microbiology, involved in extensive on-going beta glucan research since 1998 (Mode of Action of B-Glucan Immunopotentiators), continuing in 2018. The article reviews a portion of the research project sponsored by the State of Nevada and Nutritional Scientific Corporation. An additional current peer-reviewed article in Immunology Letters published in May 2004 and authored by Kenneth W. Hunter, Jr., Sally duPre and Doug Redelman is summarized in an abstract available by clicking “>MG Beta Glucan Research Abstract.” Additional research papers are available on this website by clicking on “Additional MG Glucan Research.”
The nonaggregated microparticulate beta glucan with superior immunopotentiating capabilities described is the MG glucan now processed in the NSC Laboratories by a U.S. patent protected process and patents pending. Nutritional Scientific Corporation expresses our appreciation and thanks to Dr. K. W. Hunter, Jr., Dr. Ruth Gault, M.D. Berner and Sally duPre of the Department of Microbiology and Immunology, University of Nevada School of Medicine, Reno, NV; Doug Redelman of the Department of Biology, University of Nevada, Reno, NV; and the many who have contributed to the successful research to date and who continue to make new beneficial discoveries almost daily.
Microparticulate -glucan upregulates the expression of B7.1, B7.2, B7-H1, but not B7-DC on cultured murine peritoneal macrophages
Kenneth W. Hunter, Jr. , Sally duPre’a and Doug Redelmanb
a Department of Microbiology and Immunology, University of Nevada School of Medicine, Reno, NV 89557, USA
b Department of Biology, University of Nevada, Reno, NV 89557, USA
Immunology Letters – February 2005
Abstract
-1,3-(D)-glucan from a variety of biological sources has been shown to enhance both humoral and cellular immune responses to a variety of antigens, infectious agents, and tumors. Nevertheless, its mode of action has not been fully defined. We sought to determine whether a 1–2 m diameter microparticulate form of -glucan (MG) from the yeast Saccharomyces cerevisiae could regulate expression of B7 family glycoproteins on resident peritoneal macrophages from BALB/c mice. We discovered that MG unregulated B7.2 mRNA expression and enhanced the surface membrane expression of B7.2 glycoprotein. Although B7.1 mRNA was not upregulated above constitutive levels, MG treatment enhanced B7.1 glycoprotein expression on the macrophages, albeit to a lesser extent than B7.2. At the same time, the gene and cell surface expression of B7-H1, a putative negative regulator of T cell activity, was also upregulated by MG.
The expression of B7-DC, another B7 family molecule with negative regulatory activity, was not affected by incubation with MG. This study has demonstrated that a microparticulate form of -glucan can enhance B7 co-stimulatory molecule expression on macrophages, thereby enabling these antigen-presenting cells to deliver the second signal to T-lymphocytes that express CD28. In addition, because MG also induces the expression of B7-H1, it may enable macrophages to provide a concomitant downregulatory signal to T-lymphocytes expressing PD-1 or related receptors.
Author Keywords: -Glucan; B7.1; B7.2; B7-H1; B7-DC; Macrophages; Co-stimulation
As part of an Abstract related to Microparticulate Glucan as a Vaccine Adjuvant (Jan 2003), Kenneth W. Hunter, Jr. ScD from the University of Reno School of Medicine, Dept of Microbiology reported,
“Nutritional Supply Corporation has recently developed a novel microparticulate form of beta-1,3-(D)-glucan (MG) from Saccharomyces cerevisiae…The uniform 1-2 micron diameter MG is rapidly enocytosed by APC’s (macrophages and dendritic cells), and most importantly, upregulates the expression of B7 family co-stimulatory molecules in the APC’s. Without co-stimulation, APC’s not only fail to activate T lymphocites, but they may actually induce an unresponsive state called tolerance.”
APC’s are antigen presenting cells which are essential to a proper immune response wherein T Cells are properly activated. “Enocytosis” is the process of cellular ingestion in which the plasma membrane folds inward to bring substances into the cell.
| Letters in Applied Microbiology Volume 35 Issue 4 Page 267 – October 2002 – PMID: 12358685 | |
| Preparation of microparticulate B-glucan from Saccharomyces cerevisiae for use in immune potentiation | |
| K.W. Hunter Jr, R.A. Gault and M.D. Berner | |
| Aims: To develop a method for the preparation of an immunologically active, homogeneous, nonaggregated, microparticulate -glucan-containing material from the budding yeast Saccharomyces cerevisiae. Methods and Results: Using a combination of sonication and spray-drying, a homogeneous preparation of 1-2-µ diameter -glucan-containing particles was made from alkali- and acid-insoluble yeast cell wall material. This microparticulate -glucan remained in suspension longer and, following oral administration at 0·1 mg kg Conclusions: A new sonication and spray-drying method can be employed to overcome the problem of aggregation of -glucan microparticles in aqueous media. Significance and Impact of the Study: A microparticulate form of -glucan that remains in suspension longer for pharmaceutical applications and has superior immune potentiation characteristics has been developed. | |
| Introduction -glucans are polymers of -(1,3)-D-glucose [with or without -(1,6)-D-glucose side chains] found in the cell walls of many bacteria, plants and yeasts. There is an extensive literature describing the immunomodulating effects of both water soluble and insoluble -glucans, with macrophages as the principal target cells (Reynolds et al. 1980; DiLuzio 1983; Gallin1992; Cleary et al. 1999). While various soluble and particulate -glucans have been used in pharmaceutical applications (Williams et al. 1992; Chihara 1992; Babineau et al. 1994) particulate -glucan preparations derived from the yeast Saccharomyces cerevisiae are widely used as over-the-counter nutritional supplements. Examination of several commercially available products consistently revealed a predominant ‘globular’ morphology consisting of aggregated -glucan particles ranging in size from 5 to 100-µ diameter, with some unaggregated single particles in the 1-2-µ range. While globular -glucan preparations have immune potentiating activity, it was thought that a homogeneous preparation of smaller particles would be more efficient at activating macrophages, as well as more suitable for incorporation into pharmaceutical and cosmetic formulations. As 1-2-µ diameter particles are optimally phagocytized by macrophages (Tabata and Ikada 1988), our goal was to increase the number of microparticles in this size range in the -glucan preparations. However, even after extensive grinding and sieving of dried -glucan extracted from yeast cell walls, it was discovered that the -glucan particles formed aggregates when suspended in aqueous media. Therefore, we devised a sonication and spray drying method that yielded a consistent 1-2-µ diameter particle that remained dispersed upon hydration. Although both aggregated and microparticulate glucans enhanced peritoneal macrophage activation when administered orally to mice, the microparticulate glucan was significantly better than the aggregated form. | |
| Materials and Methods | |
| Processing of yeast glucan The starting S. cerevisiae -glucan material was obtained from Nutritional Supply Corporation (Carson City, NV, USA). This material was processed from common baker’s yeast using the following procedure. Active dry yeast was added to 0·1 mol l | |
| Preparation of microparticulate -glucan Examination of the -glucan-containing powder dispersed in saline revealed aggregates ranging from 5 to 100 µ in diameter (20 µ on average). To make a uniform 1-2-µ diameter particulate preparation, the aggregated -glucan material was first hydrated in distilled H2O overnight at 4 °C. A 1·5% suspension of the hydrated material was subjected to sonic energy via a 19-mm probe utilizing a 300-V/T Sonic Dismembrator (BioLogics, Gainesville, VA, USA). Using an ultrasonic output frequency of 20 kilohertz per s at 192 watts, the glucan suspensions were sonicated on ice for 12 min (12 48-s cycles of sonication with a 12-s pause between cycles). The sonicated -glucan suspension was spray-dried using a Buchi 190 Mini-Spray Dryer (Buchi, Germany) with an inlet air temperature of 110-170 °C, an outlet air temperature of 90-120 °C and an atomizer pressure of 30-100 psi. Using flow cytometric analysis with an EPICS XL-MCL Flow Cytometer (Coulter Electronics, Hialeah, FL, USA), 1 mg of sonicated glucan consisted of 1·81 1011 microparticles. | |
| Morphology and sedimentation of the -glucan preparations -glucan preparations were suspended in normal saline and viewed with a Nikon Eclipse E400 microscope under bright-field illumination. Photomicrographs were taken with a Kodak DC digital camera. Dried -glucan samples were placed on an s.e.m. specimen holder and sputter-coated with gold to an approximately 200-Å thickness. Prepared samples were viewed on a JEOL TSM T300 Scanning Electron Microscope (s.e.m.). Suspensions of aggregated -glucan and microparticulate -glucan were prepared in distilled H2O (1·5% w/v). Each suspension was vortexed for 10 s and allowed to settle in one gravity in a 15-ml test tube for 0, 2, 5, 10, 20, 30 or 60 min. Photographs were taken of the sedimentation using a Kodak digital camera. | |
| Phagocytosis of bioparticles by peritoneal macrophages from mice treated orally with -glucan preparations Six- to eight-week old female BALB/c mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The mice were housed and cared for under an approved protocol in accordance with NIH/USDA guidelines. They were given daily oral doses of 0·1 mg kg All reagents and -glucan preparations had < 10 pg ml | |
| Results and Discussion -glucan-containing material resulting from the chemical extraction process detailed in the Methods section was examined by light microscopy after hydration in distilled H2O or saline. Although disaggregation was accomplished by sonication using the optimized conditions outlined in the Methods section (Fig. 1b), when the sonicated material was air-dried (either directly or after addition of various organic solvents such as acetone) the resulting dry material had the consistency of cardboard. This material could be ground into a fine powder with a mortar and pestle, but upon hydration in distilled H2O or saline it demonstrated significant aggregation (Fig. 1c). To overcome this re-aggregation problem, we employed a spray-drying technique. The fine powder resulting from this spray-drying process when hydrated in distilled H2O or saline resulted in a homogeneous suspension of 1-2-µ diameter particles with very few small aggregates (Fig. 1d). Interestingly, the addition of an excipient like maltodextrin did not significantly improve the process. A similar ultrasonic approach was used by Levis and Deasy (2001) to achieve particle size reduction of microcrystalline cellulose. These authors discovered that re-aggregation in aqueous media was substantially reduced by spray-drying, with or without the addition of a surfactant. Just how sonication and spray-drying alters the chemical or physical attributes of particles to mitigate against re-aggregation remains to be determined. The aggregated -glucan and the microparticles obtained following the sonication and spray-drying procedure were gold shadowed and examined with a s.e.m.. Figure 1(e) shows the morphology of a typical aggregate with a diameter of approximately 35 µ. Note that this aggregate appears to be composed of subunits in the 1-2-µ diameter size range (
-glucans bind to glucan receptors on phagocytic cells (Goldman 1988; Czop and Kay 1991; Brown and Gordon 2001) and cause these cells to become ‘activated’ (DiLuzio 1983). Earlier studies by Suzuki et al. (1990) in mice showed that oral administration of a -1,3-glucan derived from the fungus Sclerotinia sclerotiorum enhanced the phagocytic activity of peritoneal macrophages. Therefore, we compared the ability of orally administered microparticulate and aggregated -glucan preparations given at 0·1 mg kg In addition, the microparticulate -glucan was more stimulatory than the aggregated -glucan (P=0·06). Also, the number of bioparticles ingested/cell was increased over controls by both aggregated -glucan and microparticulate -glucan (P< 0·05), and macrophages from microparticulate -glucan-treated mice ingested more bioparticles/cells than macrophages from mice treated with aggregated -glucan (P=0·06). These data imply that both microparticulate and aggregated -glucan can survive transit through the gastrointestinal tract in forms capable of being absorbed and ultimately of interacting with -glucan receptors on the surfaces of resident peritoneal macrophages. It appears that the same dose of microparticulate -glucan is better able to enhance macrophage phagocytosis than aggregated -glucan. In conclusion, we have developed a new method for preparing homogeneous, nonaggregated, 1-2-µ diameter -glucan-containing particles from yeast cell walls. Compared with the aggregated form of -glucan, the -glucan microparticles remain in suspension longer for pharmaceutical applications and are more effective at enhancing phagocytosis by peritoneal macrophages following oral administration. | |
| Acknowledgements We thank Brandon Carter for technical assistance. This work was supported by an Applied Research Initiative Matching Grant from the State of Nevada and Nutritional Supply Corporation, Carson City, Nevada, NV, USA. | |
| References: | |
| • | Babineau, T.J., Marcello, P., Swails, W., Kenler, A., Bistrian, B. and Forse, R.A. (1994) Randomized phase I/II trial of a macrophage-specific immunomodulator (PPG-glucan) in high-risk surgical patients. Annals of Surgery 220, 601-609. |
| • | Bacon, J.S.D., Farmer, V.C., Jones, D. and Taylor, I.F. (1969) The glucan components of the cell wall of Baker’s yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochemical Journal 114, 557-569. |
| • | Brown, G.D. and Gordon, S. (2001) A new receptor for -glucans. Nature 413, 36-37. |
| • | Chihara, G. (1992) Recent progress in immunopharmacology and therapeutic effects of polysaccharides. Development of Biological Standards 77, 191-197. |
| • | Cleary, J.A., Kelly, G.E. and Husband, A.J. (1999) The effect of molecular weight and -1,6-linkages on priming of macrophage function in mice by (1,3)–D-glucan. Immunology and Cell Biology 77, 395-403. |
| • | Czop, J.K. and Kay, J. (1991) Isolation and characterization of -glucan receptors on human mononuclear phagocytes. Journal of Experimental Medicine 173, 1511-1520. |
| • | DiLuzio, N.R. (1983) Immunopharmacology of glucan: a broad spectrum enhancer of host defense mechanisms. Trends in Pharmacological Science 4, 344-347. |
| • | Gallin, E.K., Green, S.W. and Patchen, M.L. (1992) Comparative effects of particulate and soluble glucan on macrophages of C3H/HeN and C3H/HeJ mice. International Journal of Immunopharmacology 14, 173-183. |
| • | Goldman, R. (1988) Characteristics of the -glucan receptor of murine macrophages. Experimental Cell Research 174, 481-490. |
| • | Hata, H., Onishi, H. and Machida, Y. (2000) Preparation of CM-chitin microspheres by complexation with iron (III) in w/o emulsion and their biodisposition characteristics in mice. Biomaterials 21, 1779-1788. |
| • | Levis, S.R. and Deasy, P.B. (2001) Pharmaceutical applications of size reduced grades of surfactant co-processed microcrystalline cellulose. International Journal of Pharmaceutics 6, 25-33. |
| • | Manners, D.J., Masson, A.J. and Patterson, J.C. (1973) The structure of a b-(1,3)-D-glucan from yeast cell walls. Biochemical Journal 135, 19-30. |
| • | Reynolds, J.A., Kastello, M.D., Harrington, D.G., Crabbs, C.L., Peters, C.J., Jemski, J.V., Scott, G.H. and Di Luzio, N.R. (1980) Glucan-induced enhancement of host resistance to selected infectious diseases. Infection and Immunity 30, 51-57. |
| • | Suzuki, I., Tanaka, H., Kinoshita, A., Oikawa, S., Osawa, M. and Yadomae, T. (1990) Effect of orally administered beta-glucan on macrophage function in mice. International Journal of Immunopharmacology 12, 675-684. |
| • | Tabata, Y. and Ikada, Y. (1988) Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophages. Biomaterials 9, 356-362. |
| • | Williams, D.L., Pretus, H.A., Mcnamee, R.B., Jones, E.L., Ensley, H.W. and Browder, I.W. (1992) Development of a water-soluble, sulfated (1->3)-beta-D-glucan biological response modifier derived from Saccharomyces cerevisiae. Carbohydrate Research 235, 247-257. |
The statements in this Report have not been evaluated by the Food and Drug Administration. The products mentioned are not intended to diagnose, treat, cure or prevent any disease.
For Information about additional Free Immunition Reports, Call 888-541-3997 today
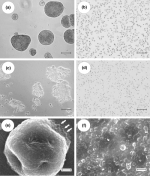 This material was determined to be a heterogeneous mixture of individual microparticles (1-2 µ in diameter) and glucan particle aggregates ranging from 5 to 100-µ diameters (
This material was determined to be a heterogeneous mixture of individual microparticles (1-2 µ in diameter) and glucan particle aggregates ranging from 5 to 100-µ diameters ( To demonstrate that the microparticulate -glucan preparation had a lower sedimentation rate, we performed the experiment shown in
To demonstrate that the microparticulate -glucan preparation had a lower sedimentation rate, we performed the experiment shown in